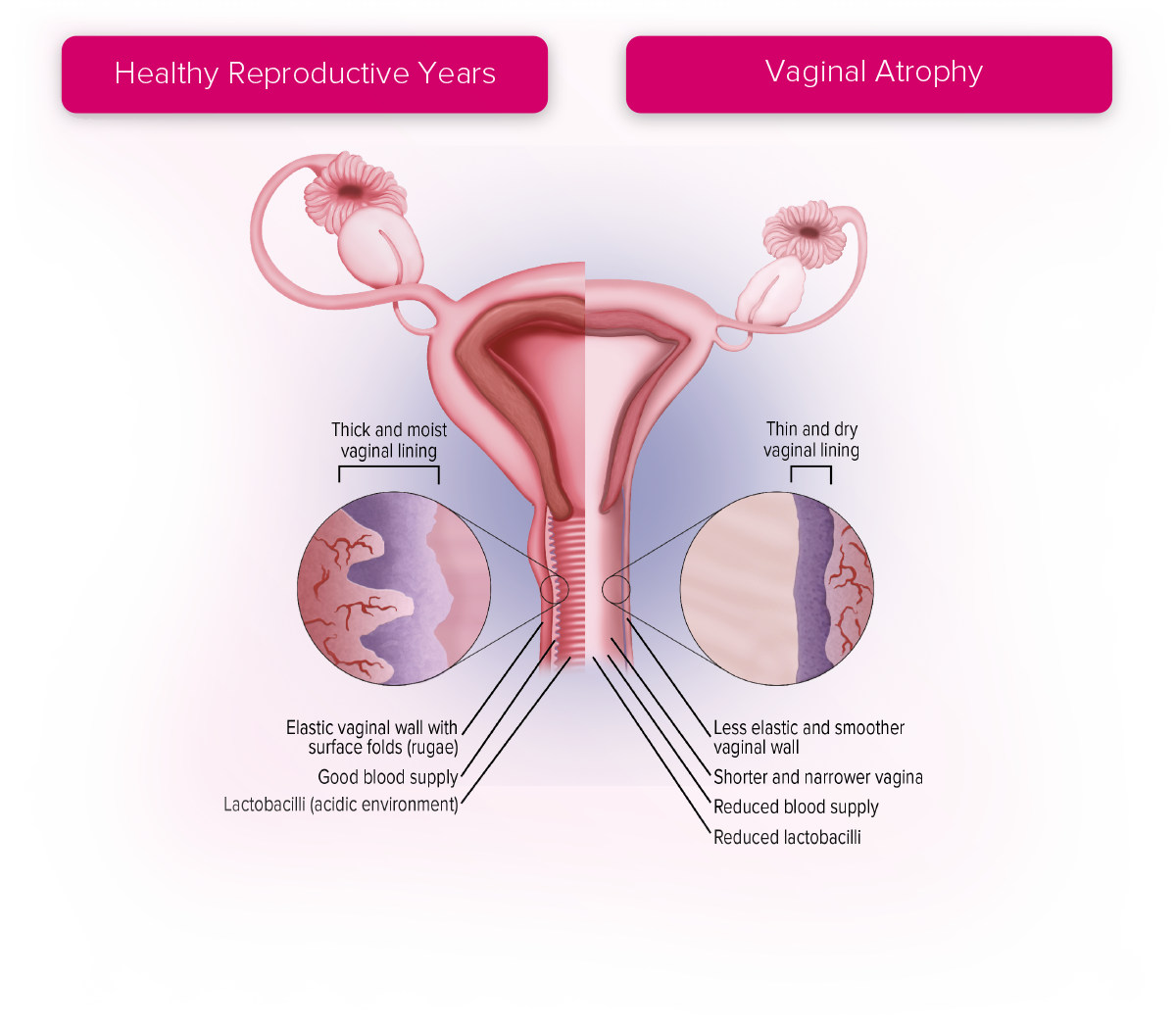
Vaginal Atrophy
Vaginal atrophy results from the natural decline in oestrogen levels and is recognised to be a progressive, long-term condition following the menopause.5
A range of terms are used to describe the genital and urinary symptoms of the menopause, including urogenital atrophy, genitourinary syndrome of menopause (GSM) and vulvovaginal atrophy.5,6,7 Vasomotor symptoms of the menopause (e.g., night sweats, hot flushes) will usually resolve without treatment, but the symptoms of vaginal atrophy do not.8 Symptoms are directly related to declining levels of oestrogen.9
UK-EST-06-24-00009 February 2025